CE Certification Parainfluenza Virus Type 2 Manufacturers Exporters – Novel Coronavirus (2019-nCoV) Nucleic Acid Test Kit (Multiple Fluorescence PCR) – INNOVITA
CE Certification Parainfluenza Virus Type 2 Manufacturers Exporters – Novel Coronavirus (2019-nCoV) Nucleic Acid Test Kit (Multiple Fluorescence PCR) – INNOVITA Detail:
Product Detail:
Innovita® 2019-nCoV IgM/IgG Test is intended for the diagnosis and epidemiological monitoring of diseases caused by the novel coronavirus (2019-nCoV). ORF1ab and N gene of 2019-nCoV are detected qualitatively from throat swabs and alveolar lavage fluid samples collected from suspected cases of pneumonia, patients with suspected cases, others who need to diagnose.
The test results of this kit are for clinical reference only. It is recommended to conduct a comprehensive analysis of the condition based on the patient’s clinical manifestations and other laboratory tests.
Principle:
This kit uses one-step reverse transcription polymerase chain reaction (RT-PCR) detection technology to target the novel coronavirus (2019-nCoV) ORF1ab gene, N gene, and human internal reference gene sequence. Specific primers and taqman probes were designed in conserved regions.
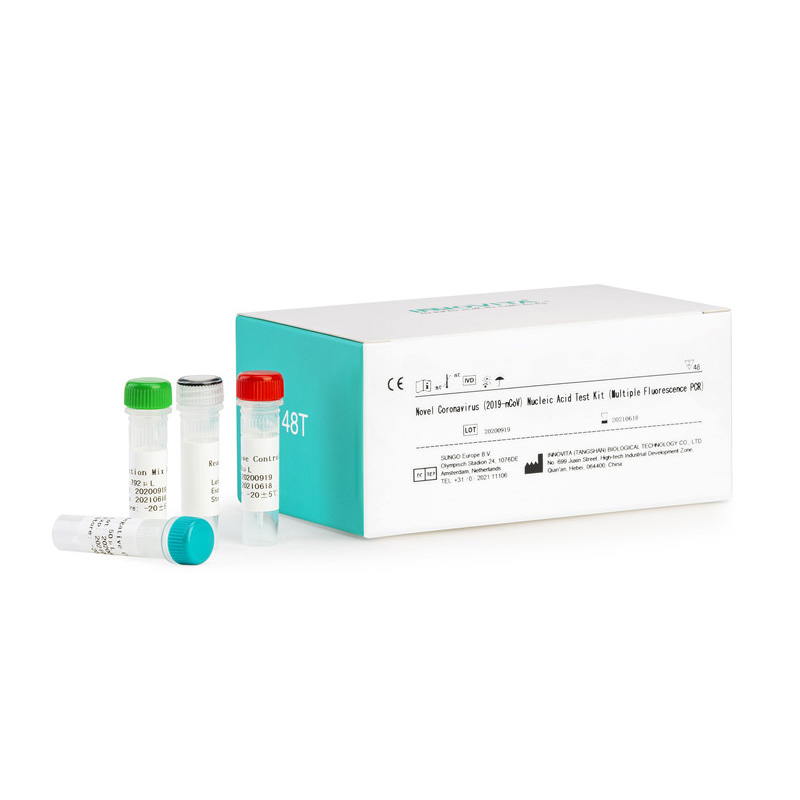
Composition:
|
Composition |
48 tests / kit |
| Reaction Mix A | 792μL×1Tube |
| Reaction Mix B | 168μL×1Tube |
| Positive Control | 50μL×1Tube |
| Negative Control | 50μL ×1Tube |
Note: 1. Different batches of reagents should not be mixed.
2. Positive controls and positive controls need not be extracted
Test Procedure:
1. Nucleic acid extraction:
Commercial RNA extraction kits are available, magnetic bead extraction and spin column extraction are recommended for this kit.
2. Preparation the Reaction Mix:
● Take out the 2019-nCoV reaction Mix A/B and keep at room temperature until unfreezing;
● Take the corresponding portions (Reaction Mix A 16.5μL/T, Reaction Mix B 3.5μL/T) and mix, and then aliquot each PCR reaction with 20μL/ tube;
● Add 5μL of RNA template or negative control or positive control, then cover the tube cap;
● Place the reaction tube in the fluorescence PCR instrument, and set the negative / positive control and sample parameters for RT-PCR reaction according to the instrument operating instructions.
● Record Sample placement order
3.RT-PCR protocols:
Recommended Settings:
|
Cycle |
Time |
Temperature(℃) |
|
|
1 |
1 |
10min |
25 |
|
2 |
1 |
10min |
50 |
|
3 |
1 |
10min |
95 |
|
4 |
45 |
10s |
95 |
|
35s |
55 |
Product detail pictures:
Related Product Guide:
We offer great strength in quality and development,merchandising,sales and marketing and operation for CE Certification Parainfluenza Virus Type 2 Manufacturers Exporters – Novel Coronavirus (2019-nCoV) Nucleic Acid Test Kit (Multiple Fluorescence PCR) – INNOVITA , The product will supply to all over the world, such as: Ethiopia, Spain, Madrid, If you have any requests, pls email Us with your detailed demands, we will give you the most wholesale Competitive Price with the Super Quality and the Unbeatable First-class Service ! We can give you the most competitive prices and high quality, because we are much more PROFESSIONAL! So please do not hesitate to contact us.
As a veteran of this industry, we can say that the company can be a leader in the industry, select them is right.



