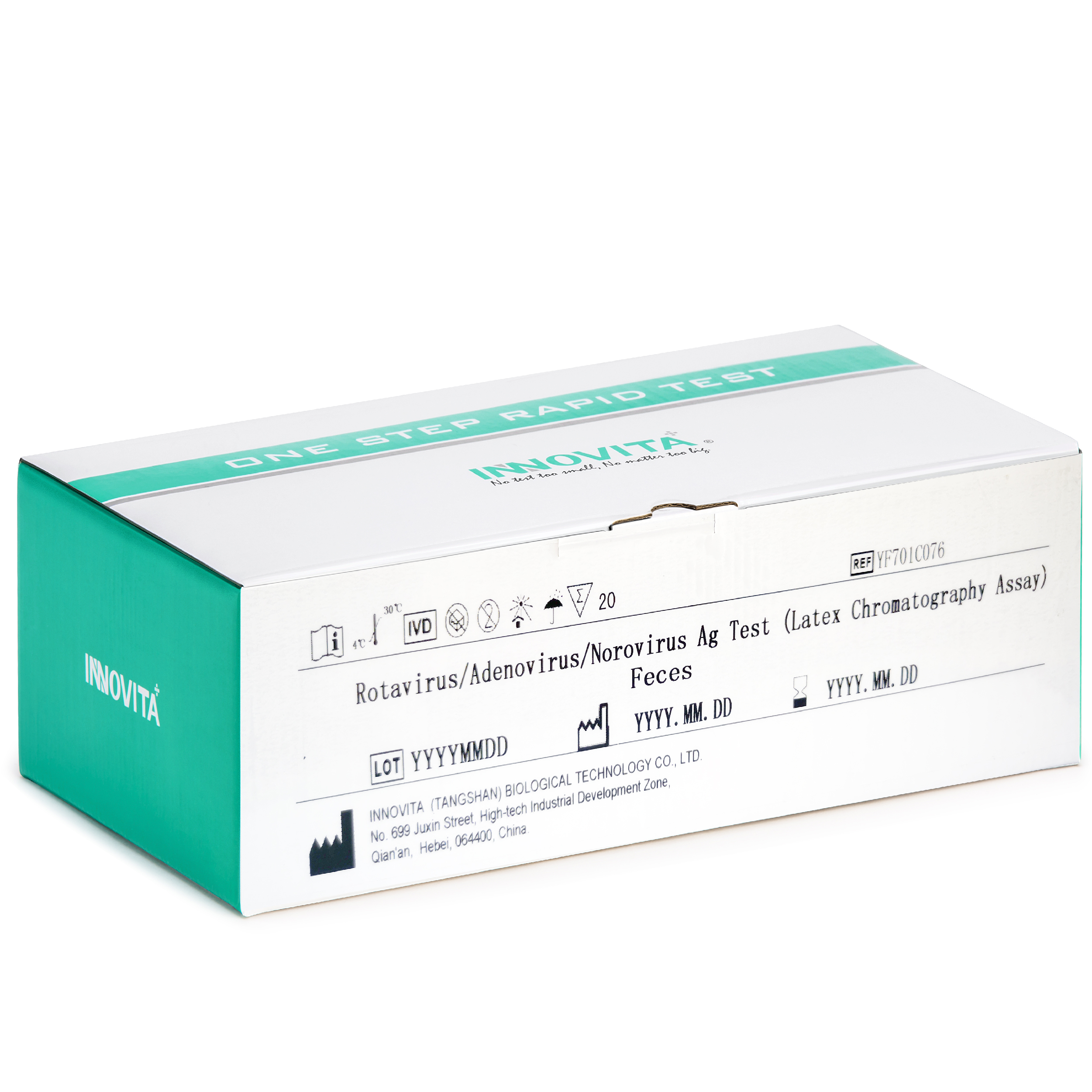
Rotavirus/Adenovirus/Norovirus Ag Test
Intended Use
The kit is intended for the direct and qualitative detection of group A rotavirus antigens, adenovirus antigens 40 and 41, norovirus (GI) and norovirus (GII) antigens in human feces specimens.
A positive test result requires further confirmation. A negative test result does not rule out the possibility of infection.
The test results of this kit are for clinical reference only. It is recommended to conduct a comprehensive analysis of the condition based on the patient's clinical manifestations and other laboratory tests.
Summary
Rotavirus (RV) is an important pathogen that causes viral diarrhea and enteritis in infants and young children worldwide. The peak of incidence is in autumn, also known as "autumn diarrhea of infants and young children". The incidence of viral diseases in infants within months and 2 years of age is as high as 62%, and the incubation period is 1 to 7 days, generally less than 48 hours, manifested by severe diarrhea and dehydration. After invading the human body, it replicates in the villous epithelial cells of the small intestine and is discharged in large quantities with feces.
Adenovirus (ADV) is a double stranded DNA virus with a diameter of 70-90nm. It is a symmetric icosahedral virus with no envelope. The virus particles are mainly composed of protein shells and core double stranded DNA. Enteric adenovirus type 40 and type 41 of subgroup F are important pathogens of viral diarrhea in human, mainly affecting infants and young children (under 4 years old). The incubation period is about 3 to 10 days. It replicates in intestinal cells and is excreted in the feces for 10 days. Clinical manifestations are abdominal pain, diarrhea, watery feces, accompanied by fever and vomiting.
Norovirus (NoV) belongs to the caliciviridae family and has 20-hedral particles with a diameter of 27-35 nm and no envelope. Norovirus is one of the main pathogens causing non-bacterial acute gastroenteritis at present. This virus is highly infectious and is mainly transmitted by contaminated water, food, contact transmission and aerosol formed by pollutants. Norovirus is the second main pathogen that causes viral diarrhea in children, and it breaks out in crowded places. Noroviruses are mainly divided into five genomes (GI, GII, GIII, GIV and GV), and the main human infects are GI, GII and GIV, among which the GII genome are the most common virus strains worldwide. Clinical or laboratory diagnostic methods of norovirus infection mainly include electron microscopy, molecular biology and immunological detection.
Composition
Specimen Collection and Handling
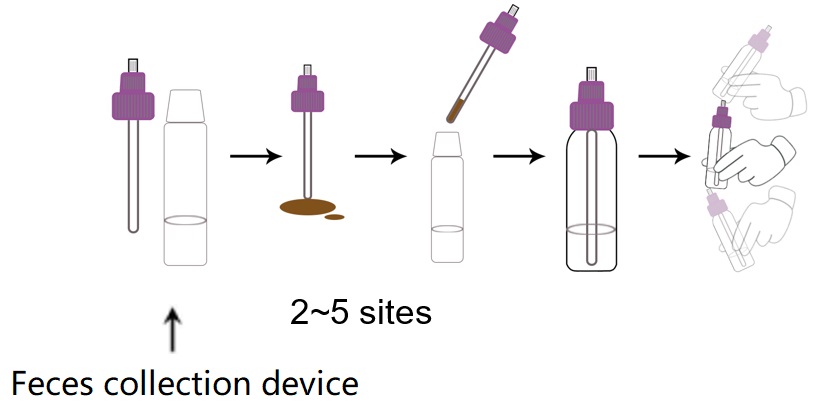
1. Collect a random feces specimen in a clean, dry receptacle.
2. Open the feces collection device by unscrewing the top and use the collection shovel to randomly
3. pierce the feces specimen in 2~5 different sites to collect around 100mg solid feces (equivalent to 1/2 of a pea) or 100μL liquid feces. Do not scoop feces specimen as this may lead to an invalid test result.
4. Ensure feces specimen is only in the grooves of the collection shovel. Excess feces specimen may lead to an invalid test result.
5. Screw on and tighten the cap onto the specimen collection device.
6. Shake the feces collection device vigorously.
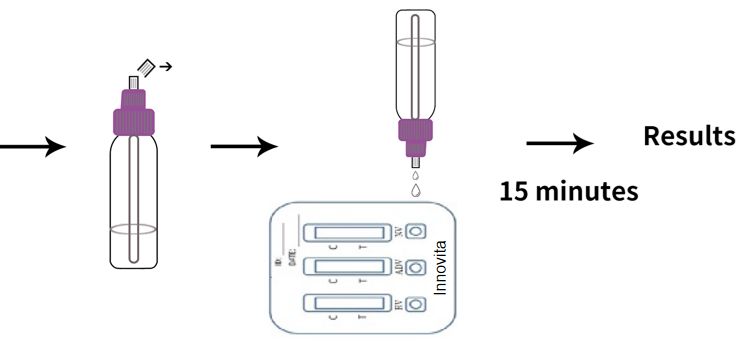
Test Procedure
1. Bring the specimen and test components to room temperature if refrigerated or frozen.
2. When you are ready to begin testing, open the sealed pouch by tearing along the notch. Remove the test from the pouch.
3. Place the test device on a clean, flat surface.
4. Position the feces collection device upright and twist off the dispenser cap.
5. Holding the feces collection device vertically, apply 80μL (around 2 drops) of the solution into the specimen well of the test device. Do not overload specimen.
6. Read the test result within 15 minutes. Do not read the result after 15 minutes.
Results Interpretation
1. Positive: The presence of two red-purple lines (T and C) within the result window indicates positive for RV/ADV/NoV antigen.
2. Negative: Only one red-purple line appearing at the control line (C) indicates negative result.
3. Invalid: If control line (C) fails to appear, no matter whether the T line is visible or not, the test is invalid. Review the procedure and repeat the test with a new test device.